806
Views & Citations10
Likes & Shares
Malignant
mixed germ cell tumor is a type of tumor that consists of two or more germ cell
tumor components. Most common combination reported is dysgerminoma and
Endodermal sinus tumor. These tumors can occur in women at any age, but peak
incidence is seen during the early 20’s. This is a case report of an 18 year
old girl with a 5 kg intra-abdominal left ovarian tumor. These tumors show
elevated levels of either alpha fetoprotein or beta hcg or both. These tumors
are chemosensitive and hence should be given chemotherapy following surgery.
Keywords: Yolk sac tumor, Immature teratoma, Mixed germ
cell tumor
INTRODUCTION
Malignant mixed germ cell tumor has two or
more germ cell tumor components. Ovarian germ cell tumor occurs most commonly
in first two decades of life and accounts for about 15 to 20% of all ovarian
neoplasms. Ovarian Malignant germ cell tumor contributes to less than 5% which
include dysgerminoma, immature teratoma, yolk sac tumor, mixed germ cell tumor
[1]. Malignant Mixed germ cell tumor represents only less than 1% of all
ovarian germ cell tumors. Most common combination reported is dysgerminoma and
Endodermal sinus tumor [2].
CASE REPORT
A 18 year old girl presented to surgical
outpatient department with the chief complaints of abdominal distention and
pain for four months duration. She complained of loss of weight and loss of
appetite. Her menstrual history revealed that she attained menarche at the age
of 12 with irregular cycles and oligomenorrhea. There was no history of
malignancies in the family.
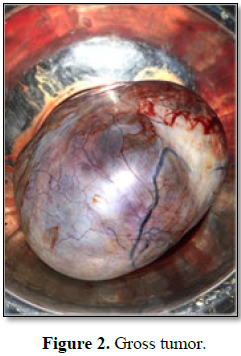
Examination of the abdomen revealed a 30 × 40
cm mobile mass, that didn't move with respiration, arising from the pelvis. The
mass occupied all quadrants of the abdomen and had variable consistency.
Ultrasonogram of the abdomen revealed a huge abdomino-pelvic mass with solid
and cystic components with non visualised left ovary and normal looking right
ovary. There was no evidence of free fluid in the abdomen. The contrast
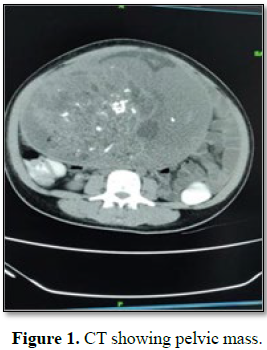
enhanced CT (Figure 1) showed
heterogeneously enhancing multiloculated solid-cystic mass lesion in the left
adnexa (13.7 × 20.9 × 23.5 cm). The cystic area was multiloculated with
enhancing thin septations with multiple central and eccentric calcification and
peripheral enhancing thin margins. Tumor markers levels were elevated (CA-125 –
265 U/ml (N<35 U/ml), alphafetoprotein (AFP) – 520 ng/ml (N<7.51 g/ml),
human chorionic gonadotropin (hcG) 2.39 ml IU/ml (Pregancy positive>25 ml U/ml)).
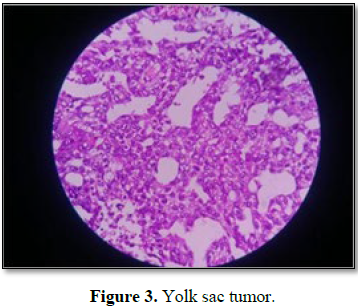
Histopathology showed germ cell origin of
ovarian tumor. The predominant component was yolk sac tumor (Figure 3)
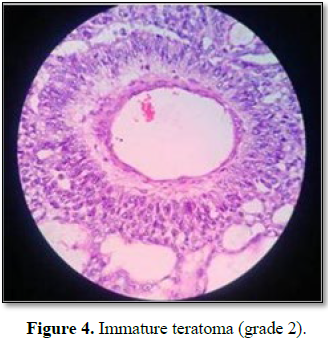
showing microcystic areas with Schiller Duvall bodies and the presence of
neuroectodermal cells suggestive of immature teratoma (Figure 4). Two
out of the twelve para-aortic lymph node were involved. Final histopathological
diagnosis was malignant mixed germ cell
tumor of ovary- Yolk sac tumor (60%) and immature teratoma- Grade 2 (40%) with
FIGO stage III A1 (II).
Patient received four cycles of bleomycin,
etoposide and cisplatin combination chemotherapy. Patient is doing well at 6
months follow up.
DISCUSSION
Ovarian tumors can occur in women at any age,
but peak incidence is seen during the early 20's. In children and adolescents,
more than 60% of ovarian neoplasms are of germ cell origin, of which
approximately 1/3 are malignant [3]. Dysgerminoma is the most common germ cell
tumor, accounting for 50% of all germ cell tumor cases [3]. The yolk sac tumor
(also known as endodermal sinus tumor) is the second most common germ cell
tumor and is common in girls with an average of 19 years [3]. Less common
tumors are embryonal carcinoma, immature teratoma, choriocarcinoma,
polyembryomoas and mixed germ cell tumors.
These tumors are usually unilateral; however
10-15% pure dysgerminomas are bilateral [3]. In our case, the combination was
yolk sac tumor and immature teratoma. A large number of malignant elements,
such as yolk sac tumor and high grade immature teratoma are often associated
with more aggressive behavior [4]. The definition of large ovarian
mass varies from those measuring more than 10 cm in diameter in preoperative
scans to those reaching above umbilicus [5]. Immature teratoma are typically (14-25
cm) larger than mature cystic teratoma.
Clinically, most patients present with
abdominal distension and pelvic pain. About 10% of patient presents with acute
abdominal pain due to rupture, hemorrhage or torsion of the ovarian mass [3].
Definite diagnosis of the tumor can only be made by histopathology.
Ultrasonography or CT is done to delineate the size and to determine the
complexity of tumors [1]. Mixed lesions may secrete either hcG, alpha
fetoprotein or both or neither of these markers, depending on the components.
The Current Royal College of Obstetricians and Gynecologist Green-top Guideline
for ovarian masses recommends determination of serum Lactate Dehydrogenase (LDH),
alpha fetoprotein and hcG in all women aged under 40 years [6]. These tumors
require urgent evaluation and treatment as the disease progress rapidly with
short tumor doubling time and spread to the peritoneum, lungs, liver and brain.
Metastatic disease has a higher probability of drug resistance and life
threatening complications including intratumoral hemorrhage.
Immunohistochemistry help in the diagnosis and development of new management
modalities [7,8].
The prognosis of germ cell tumor and sex cord
ovarian tumor is much better than epithelial ovarian cancer. Large tumor size,
unfavorable histological type and advanced stage at presentation are the poor
prognostic factors. These tumors has good prognosis because of higher number of
diagnosis at early stages and due to high chemosensitivity [9]. Mahdi et al.
[10] showed that presence of lymph node metastasis had no adverse effect on
long term outcome.
The standard treatment regimen for MOGCT with
fertility desiring is Fertility sparing surgery [11]. That is
preservation of uterus and contralateral ovary with staging procedure. Comprehensive
staging can be omitted for young patients with early stage of the disease.
Zanetta et al. [12] revealed that it is possible to restore normal gonadal
function and fertility after conservative surgery [12].
All patients except those with FIGO stage 1a
require combination therapy. Usually combination chemotherapy contains
bleomycin, etoposide and cisplatin (BEP) or vincristine, dactinomycin and
cylophosphamide (VAC), wherein three cycles for completely resected disease and
four cycle for macroscopic residual disease. Cisplatin is replaced by
carboplatin in women with renal function abnormalities. Excellent therapy has
been reported with reported with adjuvant BEP therapy. Second look surgery or removal of lesion
is indicated in case of recurrence of tumor. A study conducted by Ertas et al.
[13] showed that fertility rates were higher for those who received adjuvant
chemotherapy after fertility sparing surgery. The POMB/ACE regimen (cisplatin,
vincristine, methotrexate and bleomycin (POMB) alternating with actinomycin D,
cyclophosphamide and etoposide (ACE) was designed to reduce risk of drug
resistance [14]. About 75% of MOGCT recurrences occur within first year, so
follow up every 4-8 weeks [15]. Follow up should include tumor markers, chest
X-ray and CT imaging after 3 months of therapy.
CONCLUSION
Malignant mixed germ tumor of ovary with
components of yolk sac tumor and high grade immature teratoma are extremely
rare and highly aggressive. Fertility sparing surgery with adjuvant
chemotherapy is treatment of choice for adolescent girls presenting with
ovarian mass.
1. Lim FK, Chanrachakul B, Chong SM, Ratnam SS (1998)
Malignant ovarian germ cell tumors: Experience in the National university
Hospital of Singapore. Ann Aead Med Singapore 27: 657-661.
2. Gershenson DM, Junco GD, Copeland LJ, Rutledge FN
(1984) Mixed germ cell tumors of the ovary. Obstet Gynecol 64: 200-206.
3. Tiwary B, Sinha HH, Pandey VK (2015) Malignant
mixed germ cell tumor of ovary: A rare case report. Int J Reprod Contracept
Obstet Gynecol 4: 511-513.
4. Talerman A (2002) Germ cell tumors of the ovary.
In: Kurman RJ, ed. Blaustein's pathology of the female genital tract. 5th
Edn. New York, NY: Springer, p: 1391.
5. Singla DK, Kansal R, Bansal I, Thanu G, Agrawal N
(2014) Laproscopic management of a giant ovarian cyst. Asian Pac J Health Sci
1: 43-46.
6. Royal College of Obstetricians and Gynecologists
(2011) Management of suspected ovarian masses in premenopausal women. Green-top
Guideline No.62. London: RCOG.
7. de Joliniere JB, Ben Ali N, Fadhloui A, Dubuisson
JB, Guillou L, et al. (2014) Two case reports of a malignant germ cell tumor of
ovary and a granulosa cell tumor: Interest of tumoral immunochemistry in the
identification and management. Front Oncol 4: 9.
8. Trinh DT, Shibata K, Hirosawa T, Umezu T, Mizuno M,
et al. (2012) Diagnostic utility of CD117, CD133, SALL4, OCT4, TCL1 and
glypican 3 in malignant germ cell tumors of the ovary. J Obstet Gynecol Res;
385: 841-848.
9. Park JY, Kim DY, Suh DS, Kim JH, Kim YM, et al.
(2017) Analysis of outcomes and prognostic factors after fertility sparing
surgery in malignant ovarian germ cell tumors. Gynecol Oncol 145: 513-518.
10. Mahdi H, Swensen RE, Hanna R, Kumar S, Ali-Fehmi R,
et al. (2011) Prognostic impact of lymphadenectomy in clinically early stage
malignant germ cell tumor of the ovary. Br J Cancer 105: 493-497.
11. Zhang N, Chen R, Hua K, Zhang Y (2017) A
retrospective study of reproductive outcomes after fertility-sparing surgery
and postoperative adjuvant chemotherapy in malignant ovarian germ cell tumors
and sex cord-stromal tumors. J Ovarian Res 10: 52.
12. Zanetta G, Bonazzi C, Cantu M, Binidagger S,
Locatelli A, et al. (2001) Survival and reproductive function after treatment
of malignant germ cell ovarian tumors. J Clin Oncol 19: 1015-1020.
13. Ertas IE, Taskin S, Goklu R, Bilgin M, Goc G, et
al. (2014) Long term oncological and reproductive outcomes of fertility sparing
cytoreductive surgery in females aged 25 years and younger with malignant
ovarian germ cell tumors. J Obstet Gyenecol Res 40: 797-805.
14. Bower M, Newlands ES, Holden L, Rustin GJS, Begent
RHJ (1997) Treatment of men with metastatic non-seminomatous germ cell tumors
with cyclical POMB/ACE chemotherapy. Ann Oncol 8: 477-483.
15. Colombo N, Peiretti M, Castiglione M; ESMO
Guidelines Working Group (2009) Non epithelial ovarian cancer: ESMO clinical
recommendations for diagnosis, treatment and follow up. Ann Oncol 20: 24-26.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)